Gravimetric Analysis - GRE Subject Test: Chemistry
Card 0 of 4
Household vinegar contains the organic compound acetic acid with chemical formula,  . If a
. If a  vinegar sample contains
vinegar sample contains  of acetic acid, calculate the percent (mass/mass) of the
of acetic acid, calculate the percent (mass/mass) of the  in the vinegar sample.
in the vinegar sample.
Density of vinegar is the following:

Household vinegar contains the organic compound acetic acid with chemical formula, 



Density of vinegar is the following:
Convert the moles of 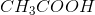 to grams:
to grams:

To calculate the percentage of  in the vinegar we need to use the following formula:
in the vinegar we need to use the following formula:

Therefore,

Convert the moles of 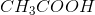
To calculate the percentage of 
Therefore,
Compare your answer with the correct one above
Household vinegar contains the organic compound acetic acid with chemical formula,  . If a
. If a  vinegar sample contains
vinegar sample contains  of acetic acid, calculate the percent (mass/mass) of the
of acetic acid, calculate the percent (mass/mass) of the  in the vinegar sample.
in the vinegar sample.
Density of vinegar is the following:

Household vinegar contains the organic compound acetic acid with chemical formula, 



Density of vinegar is the following:
Convert the moles of 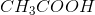 to grams:
to grams:

To calculate the percentage of  in the vinegar we need to use the following formula:
in the vinegar we need to use the following formula:

Therefore,

Convert the moles of 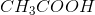
To calculate the percentage of 
Therefore,
Compare your answer with the correct one above
Household vinegar contains the organic compound acetic acid with chemical formula,  . If a
. If a  vinegar sample contains
vinegar sample contains  of acetic acid, calculate the percent (mass/mass) of the
of acetic acid, calculate the percent (mass/mass) of the  in the vinegar sample.
in the vinegar sample.
Density of vinegar is the following:

Household vinegar contains the organic compound acetic acid with chemical formula, 



Density of vinegar is the following:
Convert the moles of 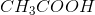 to grams:
to grams:

To calculate the percentage of  in the vinegar we need to use the following formula:
in the vinegar we need to use the following formula:

Therefore,

Convert the moles of 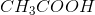
To calculate the percentage of 
Therefore,
Compare your answer with the correct one above
Household vinegar contains the organic compound acetic acid with chemical formula,  . If a
. If a  vinegar sample contains
vinegar sample contains  of acetic acid, calculate the percent (mass/mass) of the
of acetic acid, calculate the percent (mass/mass) of the  in the vinegar sample.
in the vinegar sample.
Density of vinegar is the following:

Household vinegar contains the organic compound acetic acid with chemical formula, 



Density of vinegar is the following:
Convert the moles of 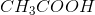 to grams:
to grams:

To calculate the percentage of  in the vinegar we need to use the following formula:
in the vinegar we need to use the following formula:

Therefore,

Convert the moles of 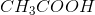
To calculate the percentage of 
Therefore,
Compare your answer with the correct one above