Reduction Reactions - MCAT Biological and Biochemical Foundations of Living Systems
Card 1 of 8
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Tap to reveal answer
Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 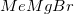 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

← Didn't Know|Knew It →
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Tap to reveal answer
Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 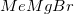 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

← Didn't Know|Knew It →
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Tap to reveal answer
Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 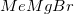 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

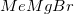
← Didn't Know|Knew It →
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Tap to reveal answer
Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 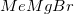 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

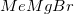
← Didn't Know|Knew It →
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Tap to reveal answer
Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 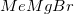 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

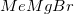
← Didn't Know|Knew It →
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Tap to reveal answer
Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 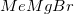 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

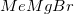
← Didn't Know|Knew It →
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Tap to reveal answer
Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 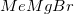 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

← Didn't Know|Knew It →
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Tap to reveal answer
Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 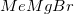 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

← Didn't Know|Knew It →