Enthalpy - SAT Subject Test in Chemistry
Card 0 of 4

Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce
 .
.
Hence, the enthalpy change is 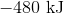 .
.
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce

Hence, the enthalpy change is 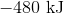
Compare your answer with the correct one above

Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce
 .
.
Hence, the enthalpy change is 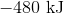 .
.
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce

Hence, the enthalpy change is 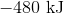
Compare your answer with the correct one above

Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce
 .
.
Hence, the enthalpy change is 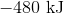 .
.
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce

Hence, the enthalpy change is 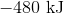
Compare your answer with the correct one above

Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce
 .
.
Hence, the enthalpy change is 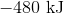 .
.
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce

Hence, the enthalpy change is 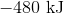
Compare your answer with the correct one above
