Hydrogen Bonding - SAT Subject Test in Chemistry
Card 0 of 4
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 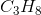 .
.
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 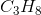
Compare your answer with the correct one above
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 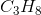 .
.
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 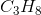
Compare your answer with the correct one above
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 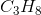 .
.
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 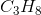
Compare your answer with the correct one above
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 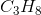 .
.
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 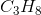
Compare your answer with the correct one above