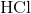Other Compound Concepts - SAT Subject Test in Chemistry
Card 0 of 4
Which of the following are strong electrolytes?
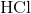




Which of the following are strong electrolytes?
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes.  is a strong acid.
is a strong acid.  is a salt.
is a salt.  is a salt.
is a salt.  is a weak acid.
is a weak acid.  is a weak base. Therefore the answer is three.
is a weak base. Therefore the answer is three.
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes. 




Compare your answer with the correct one above
Which of the following are strong electrolytes?
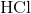




Which of the following are strong electrolytes?
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes.  is a strong acid.
is a strong acid.  is a salt.
is a salt.  is a salt.
is a salt.  is a weak acid.
is a weak acid.  is a weak base. Therefore the answer is three.
is a weak base. Therefore the answer is three.
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes. 




Compare your answer with the correct one above
Which of the following are strong electrolytes?
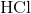




Which of the following are strong electrolytes?
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes.  is a strong acid.
is a strong acid.  is a salt.
is a salt.  is a salt.
is a salt.  is a weak acid.
is a weak acid.  is a weak base. Therefore the answer is three.
is a weak base. Therefore the answer is three.
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes. 




Compare your answer with the correct one above
Which of the following are strong electrolytes?
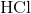




Which of the following are strong electrolytes?
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes.  is a strong acid.
is a strong acid.  is a salt.
is a salt.  is a salt.
is a salt.  is a weak acid.
is a weak acid.  is a weak base. Therefore the answer is three.
is a weak base. Therefore the answer is three.
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes. 




Compare your answer with the correct one above