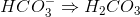Identifying and Defining Acids and Bases
Help Questions
SAT Subject Test in Chemistry › Identifying and Defining Acids and Bases
Questions 1 - 1
1
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
Explanation