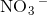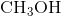Ionic Bonds
Help Questions
SAT Subject Test in Chemistry › Ionic Bonds
How many of the following compounds are ionic?
Two
Zero
One
Three
All five
Explanation
Ionic compounds are chemical compounds made of charged ions held together by forces called ionic bonding. Ionic bonds are formed by transfers of valance electrons, which create charged atoms (ions) which are attracted to each other because they have opposite charges. This is not be confused with covalent bonding, in which atoms form an attraction by sharing electrons.