All AP Chemistry Resources
Example Questions
Example Question #111 : Reaction Types
A 1M solution of a monoprotic acid has a pH of 4.6. What is the 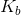
In order to find the base dissociation constant for the conjugate base, we can start by finding the acid dissociation constant for the acid. Since a 1M solution of the acid has a pH of 4.6, we can find the proton concentration of the solution.
Since the acid is monoprotic, we can set the following equilibrium expression equal to its acid dissociation constant.
We can see that, since the acid is monoprotic, the concntration of protons will be equal to the concentration of the acid anion. The final concentration of the acid molecule will be equal to the initial concentration, minus the amount of protons formed. Using these values, we can solve for the equilibrium constant for the acid.
Now that we have the acid dissociation constant, we can find the conjugate base's dissociation constant by setting the product of the two values equal to the autoionization of water.
Example Question #21 : P H And Poh Of Strong Acids And Bases
Would H2SO4 or HNO3 produce a more acidic solution?
H2SO4 since it has a higher pKa
H2SO4 since it has a lower pKa
HNO3 since it has a higher pKa
HNO3 since it has a lower pKa
H2SO4 since it has a lower pKa
Both are strong acids, but H2SO4 is bivalent, realeasing 2 protons for each molecule dissolved in solution. Further, a more acidic solution would have a lower pKa.
Example Question #21 : P H And Poh Of Strong Acids And Bases
Which of the following species will not be present in an aqueous solution of 
All of these will be present in the solution
The hydroxide ion (OH–) is a strong base and therefore would not be present in an acidic solution. Protons, the hydronium ion, and water will all be present in relatively large amounts within the solution.
Example Question #982 : Ap Chemistry
Which of the following anions is the strongest base?
Weaker acids dissociate less in water and therefore, reverse reaction is favored in




Certified Tutor
All AP Chemistry Resources



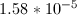

![pH=-\log[H^+]\rightarrow [H^+]=10^{-pH}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/121717/gif.latex)
![\small [H^{+}] = 10^{-4.6} = 2.51*10^{-5}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94835/gif.latex)
![\small K_{a} = \frac{[H^{+}][A^{-}]}{[HA]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/121718/gif.latex)
![K_a= \frac{[2.51*10^{-5}]^{2}}{[1-2.51*10^{-5}]} = 6.31*10^{-10}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94836/gif.latex)