All AP Physics 1 Resources
Example Questions
Example Question #1 : Coulomb's Law
Two protons are on either side of an electron as shown below:

The electron is 30 µm away from the proton on its left and 10 µm away from the proton on its right. What is the magnitude and direction of the net electric force acting on the electron?
A proton has a charge of






The net force on the electron is the sum of the forces between the electron and each of the protons:
These forces are given by Coulomb's law:
Using the numbers given, we get:
Because opposite charges attract, 

Therefore, the net force is
Because this value is positive, the direction is rightward.
Example Question #2 : Coulomb's Law
Charges A and B are placed a distance of 




What is the ratio of 

This question is very simple if you realize that the force experienced by both charges is equal.
The definition of the two electrostatic forces are given by Coulomb's law:
In this question, we can rewrite this equation in terms of our given system.
It doesn’t matter if the charges of the two particles are different; both particles experience the same force because the charges of both particles are accounted for in the electrostatic force equation (Coulomb's law). This conclusion can also be made by considering Newton's third law: the force of the first particle on the second will be equal and opposite the force of the second particle on the first.
Since the forces are equal, their ratio will be 
Example Question #2 : Coulomb's Law
An excess charge of 



Two principal realizations help with solving this problem, both derived from Gauss’ law for electricity:
1) The excess charge on an ideal conducting sphere is uniformly distributed over its surface
2) A uniform shell of charge acts, in terms of electric force, as if all the charge were contained in a point charge at the sphere’s center
With these realizations, an application of Coulomb’s law answers the question. If 

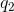
In this equation, 



Using the given values in this equation, we can calculate the generated force:
Example Question #1 : Coulomb's Law
If the distance between two charged particles is doubled, the strength of the electric force between them will __________.
be halved
double
be quartered
remain unchanged
quadruple
be quartered
Coulomb's law gives the relationship between the force of an electric field and the distance between two charges:
The strength of the force will be inversely proportional to the square of the distance between the charges.
When the distance between the charges is doubled, the total force will be divided by four (quartered).
Example Question #1 : Coulomb's Law
If we have 2 charges, 








Use Coulomb's Law
Plug in known values and solve.
A negative value for electric force indicates an attractive force. This makes sense since our two charges have opposite signs. Since we're asked for magnitude, all answer choices are positive.
Example Question #2 : Coulomb's Law
If we have 2 charges, 








Use Coulomb's law.
Plug in known values and solve.
Note that this force is positive, which means it's repulsive.
Example Question #3 : Coulomb's Law
If we have 2 charges, 








Use Coulomb's law.
Plug in known values and solve.
Note that this force is positive, which means that it's repulsive.
Example Question #1 : Coulomb's Law
If we have 2 charges, 








Use Coulomb's law.
Plug in known values and solve.
Note that the force between two charges of the same sign (both positive or both negative) is positive. This indicates the force is repulsive, which makes sense since both charges are positive.
Example Question #1 : Coulomb's Law
Two point charges, 


The force of attraction/repulsion between two point charges is given by Coulomb's Law:
If the charges are of like sign, then there well be a repulsive force between the two. Alternatively, if the net force is positive, it is repulsive; if it is negative, it is attractive.
Therefore, the force of repulsion between the two charges is:
Example Question #6 : Coulomb's Law
Two point charges, 



The force of attraction/repulsion between two point charges is given by Coulomb's Law:
If the charges are of like sign, then there well be a repulsive force between the two.
Work is given as the dot product of force and distance. However, in this case, force is also dependent on distance.
The amount of work required to move a charge an incremental distance, 
The negative sign in this case is to account for repulsion.
The total work to change distances between charges can then be found by taking the integral with respect to distance:
Since 
Certified Tutor
All AP Physics 1 Resources