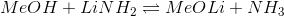All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Acid Base Reaction
For which of the following acid-base reactions will the equilibrium lie on the left side?
Possible Answers:

Correct answer:
Explanation:
The pKa value indicates how strong an acid is, and acid strength increases as pKa decreases. The side of a reaction with a lower pKa is going to dissociate more, pushing the equilibrium over to the other side. The equilibrium will thus lie on the side with the HIGHER pKa.
Since the pKa of acetic acid (4.76) is higher than the pKa of trifluoroacetic acid (0), the reaction will shift to the left to reach equilibrium.
Christy
Certified Tutor
Certified Tutor
Northern Michigan University, Bachelor in Arts, Graphic Design. Northern Michigan University, Bachelor of Science, Premedicine.
All GRE Subject Test: Chemistry Resources
Popular Subjects
SSAT Tutors in Houston, French Tutors in Los Angeles, MCAT Tutors in New York City, GMAT Tutors in Seattle, Biology Tutors in Washington DC, LSAT Tutors in Houston, MCAT Tutors in Denver, Physics Tutors in New York City, Biology Tutors in Denver, Chemistry Tutors in Philadelphia
Popular Courses & Classes
LSAT Courses & Classes in Miami, SAT Courses & Classes in Phoenix, GRE Courses & Classes in Los Angeles, GMAT Courses & Classes in Chicago, SSAT Courses & Classes in Philadelphia, LSAT Courses & Classes in Denver, GRE Courses & Classes in Atlanta, SAT Courses & Classes in San Francisco-Bay Area, MCAT Courses & Classes in Washington DC, ISEE Courses & Classes in San Francisco-Bay Area
Popular Test Prep
GMAT Test Prep in Boston, GRE Test Prep in Washington DC, GRE Test Prep in Dallas Fort Worth, SAT Test Prep in Los Angeles, ISEE Test Prep in Los Angeles, SAT Test Prep in Houston, GMAT Test Prep in Denver, MCAT Test Prep in Boston, SAT Test Prep in Philadelphia, GRE Test Prep in Atlanta