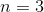All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Help With Alkene Synthesis

What is the value of 
Possible Answers:
Correct answer:
Explanation:
Huckel's rule states that an aromatic compound must have 
If 4n+2=18, then n=4.
Christy
Certified Tutor
Certified Tutor
Northern Michigan University, Bachelor in Arts, Graphic Design. Northern Michigan University, Bachelor of Science, Premedicine.
All GRE Subject Test: Chemistry Resources
Popular Subjects
LSAT Tutors in San Diego, ISEE Tutors in San Diego, SAT Tutors in San Diego, English Tutors in Seattle, Statistics Tutors in New York City, LSAT Tutors in Denver, ISEE Tutors in Washington DC, Biology Tutors in Denver, Chemistry Tutors in Miami, Algebra Tutors in Chicago
Popular Courses & Classes
ISEE Courses & Classes in Houston, SSAT Courses & Classes in New York City, SAT Courses & Classes in Seattle, MCAT Courses & Classes in Miami, ISEE Courses & Classes in Los Angeles, SAT Courses & Classes in Philadelphia, GRE Courses & Classes in Dallas Fort Worth, LSAT Courses & Classes in San Diego, SSAT Courses & Classes in San Francisco-Bay Area, SSAT Courses & Classes in Houston