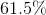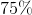All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #41 : Analytical Chemistry
Household vinegar contains the organic compound acetic acid with chemical formula, 



Density of vinegar is the following:
Possible Answers:
Correct answer:
Explanation:
Convert the moles of 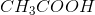
To calculate the percentage of 
Therefore,
Kimberly
Certified Tutor
Certified Tutor
University of Connecticut, Bachelor of Chemistry, Chemistry. University of Connecticut, Doctor of Philosophy, Organic Chemist...
Leann
Certified Tutor
Certified Tutor
Georgia Institute of Technology-Main Campus, Engineer, Systems Engineering. University of South Florida-Main Campus, Masters ...
All GRE Subject Test: Chemistry Resources
Popular Subjects
SSAT Tutors in Chicago, Spanish Tutors in San Francisco-Bay Area, SSAT Tutors in Dallas Fort Worth, MCAT Tutors in Atlanta, Biology Tutors in Chicago, Statistics Tutors in Los Angeles, Chemistry Tutors in Denver, Calculus Tutors in Chicago, LSAT Tutors in San Francisco-Bay Area, MCAT Tutors in Philadelphia
Popular Courses & Classes
ACT Courses & Classes in Chicago, LSAT Courses & Classes in Los Angeles, SSAT Courses & Classes in Philadelphia, SSAT Courses & Classes in Phoenix, LSAT Courses & Classes in Chicago, SAT Courses & Classes in New York City, MCAT Courses & Classes in Chicago, ISEE Courses & Classes in Chicago, ACT Courses & Classes in Denver, SSAT Courses & Classes in San Diego