All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #4 : Help With Lewis Diagrams
Which answer option correctly depicts the Lewis dot structure of sodium chloride?






When drawing a Lewis dot structure, we are always trying to reach an electron count where all atoms involved are stable and (usually) have full octets. We are also trying to estabilsh a structure in which we have the smallest formal charge possible. The general rule is first to draw out all of the elements involved and their valence electrons, then start piecing them together trying to reduce the formal charge and get all elements involved to an octet. There are a couple exceptions to the octet rule.
Sodium and chlorine form an ionic bond, meaning that one atom will donate an electron and the other will receive it. This gives each atom a charge. Chlorine has seven valence electrons, while sodium has one valence electron. For each atom to arrive at an octet, sodium will need to lose one electron and chlorine will need to gain one electron. This would give chlorine a negative charge, and sodium a positive charge.
Thus, the answer is a sodium with a positive charge (due to one lost electron) and a chlorine with eight electrons and a negative charge (due to one electron gained).
Example Question #21 : Molecules And Compounds
What is the Lewis dot structure for 






When drawing a Lewis dot structure, we are always trying to reach an electron count where all atoms involved are stable and (usually) have full octets. We are also trying to estabilsh a structure in which we have the smallest formal charge possible. The general rule is first to draw out all of the elements involved and their valence electrons, then start piecing them together trying to reduce the formal charge and get all elements involved to an octet. There are a couple exceptions to the octet rule.
In this case, boron actually has an incomplete octet. Though there are resonance forms in which boron has a full octet, when you calculate the formal charge of these configurations it will not be zero.
Example Question #1 : Diagrams And Geometry
Which of the following molecules will have the largest bond angle?
According to VSEPR theory, atoms will orient themselves in order to be as far away from neighboring atoms as possible in a molecule. This theory helps predict the geometry that molecules will take, as well as the bond angles between atoms.

All of the other given moleules will have bond angles less than 180 degrees.
Example Question #2 : Vsepr Geometry
Which of the following is not the correct geometric configuration for the given molecule?




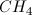

Recall the following relationships between geometry and number of pairs of electrons on the central atom.
2: linear
3: trigonal planar
4: tetrahedral
5: trigonal bipyriamidal
6: octahedral
To visualize the geometry, we need to think of how many electron pairs are on the central atom. Drawing Lewis dot diagrams may be helpful here. None of the answer choices has lone central electron pairs, with the exception of water, so the number of atoms bound to the central atom is the same as the number of central electron pairs.
The only one that does not match up with the correct geometry is SF6, which is actually octahedral since it has six central electron pairs. In a water molecule, the central oxygen has six valence electrons, plus one from each bond with hydrogen, for a total of eight central electrons and four central electron pairs. So, this geometry is a variation on the tetrahedral form (bent), in which two central electron pairs are not bound.
Example Question #2 : Molecular And Electronic Geometries
Which of the following molecules exhibits a trigonal pyramidal geometry?
The trigonal pyramidal geometry is implemented by molecules in which the central atom has three atoms and a lone pair attached. 



Example Question #1 : Molecular And Electronic Geometries
Which of the following molecules has an electronic geometry that is the same as its molecular geometry?
Electronic and molecular geometries are only the same when there are no lone pairs around the central atom in the molecule. 
Example Question #2 : Molecular And Electronic Geometries
What is the molecular shape of 
Trigonal pyramidal
Trigonal bipyramidal
Octahedral
Trigonal planar
Tetrahedral
Trigonal pyramidal
NH3 is trigonal pyramidal because it has 4 electron domains, one of them being a lone pair of electrons and the other three being H atoms. When this is arranged in a three-dimensional space, it is trigonal pyramidal in shape.
Certified Tutor
Certified Tutor
All GRE Subject Test: Chemistry Resources













