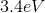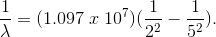All GRE Subject Test: Physics Resources
Example Questions
Example Question #21 : Gre Subject Test: Physics
Which of the following wavelengths could be used to measure the position of an electron with the greatest accuracy?
Infared
Red light
Gamma-rays
Radio
X-ray
Gamma-rays
The Heisenberg Uncertainty principle states that
Therefore, the smallest wavelength will have the lowest uncertainty in the position. Thus, we must pick the regime of options given which has the lowest wavelength. That is gamma-ray.
Example Question #1 : Quantum Mechanics And Atomic Physics
What wavelength will result in the most accurate measurement of the momentum of an electron?
Red light
Gamma-ray
X-ray
Radio
Infrared
Radio
The Heisenberg Uncertainty Principle states that:

Example Question #2 : Quantum Mechanics And Atomic Physics
If a ground state particle is in a one-dimension square well, where is the probability of finding the particle equal to zero?
None of these

At the boundary.
In the middle.

At the boundary.
This is a fundamental concept question. For a ground state particle, the only place where the probability is equal to zero is at the boundary because the particle cannot be found there. He has to be in the box (not unlike a cat).
Example Question #1 : Atomic Properties
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the 



However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.
Example Question #2 : Atomic Properties
What color will be emitted in the 

Red
Green
Blue
Violet
Yellow
Violet

Here, 




By inverting, 
Certified Tutor
Certified Tutor
All GRE Subject Test: Physics Resources