All MCAT Physical Resources
Example Questions
Example Question #3 : Buffers
Which of the following would be a good buffer solution?
A good buffer solution consists of a weak acid with its conjugate base, or a weak base and its conjugate acid. A strong acid or base will never be a good buffer. We will go through each answer choice in detail.




Example Question #445 : High School Chemistry
Which of the following pairs cannot constitute a buffer system?
A buffer system is composed of one of the following scenarios:
1. A weak base with its conjugate acid.
2. A weak acid with its conjugate base.
The only option that does not qualify is the nitrate, nitrite pair. A difference of one oxygen does not make a buffer system.
Example Question #1 : Infrared (Ir) Spectroscopy
An IR spectroscopy reading of a compound shows a large absorption at 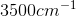
Aldehyde
Alcohol
Amide
Amine
Alcohol
Compounds with an alcohol group show absorptions in an IR sprectrum from 








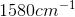



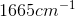
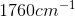
Example Question #11 : Biochemistry, Organic Chemistry, And Other Concepts
Consider the following solutions.
Solution A: 1M sodium chloride solution
Solution B: 1M calcium nitrate solution
Solution C: 1M sucrose solution
Equal volumes of the solutions are combined and the mixture is added to a distillation column. Which of the following solutions will separate first?
Solution A
Solution C
These solutions cannot be separated via distillation
Solution B
Solution C
Distillation is a process of separating a liquid from solutes or other liquids. It utilizes the boiling point differences to separate substances. A substance with a low boiling point will evaporate first in a distillation column and will be isolated first. The question is asking which solution will be isolated first; therefore, we need to figure out which solution has the lowest boiling point. Recall that the boiling point of a solution is elevated when there are more solutes present in the solution. Sodium chloride (

Example Question #11 : Biochemistry, Organic Chemistry, And Other Concepts
Small differences in boiling point require the use of __________ distillation and large differences in boiling point require the use of __________ distillation.
simple . . . simple
fractional . . . simple
simple . . . fractional
fractional . . . fractional
fractional . . . simple
There are two types of distillation. Simple distillation is used to separate molecules that have very different boiling points. Fractional distillation is used to separate molecules with small differences in boiling points. Fractional distillation is often used if the difference between boiling points is less than 
Example Question #2 : Purification Techniques
Which of the following mixtures can be separated using fractional distillation (boiling points of each substance given in 
I. Chloroform (62.2) and 
II. Iodine (184.3) and mercury (356.9)
III. Nitric acid (120) and sulfuric acid (310)
II and III
II only
I, II, and III
I only
I, II, and III
Distillation is used to separate molecules with different boiling points. Simple distillation is used to separate molecules with vastly different boiling points. Fractional distillation, on the other hand, is a refined form of simple distillation that can be used to separate molecules with similar boiling points. Note that fractional distillation can separate molecules with either different or similar boiling points; therefore, fractional distillation can be used to separate any of the given mixtures.
Example Question #1 : Distillation
Which of the following conditions will result in the greatest increase in the rate of distillation of a substance?
Decreasing the atmospheric pressure
Decreasing the temperature
Decreasing the mole fraction of the substance
Decreasing the vapor pressure
Decreasing the atmospheric pressure
Rate of distillation is increased when the ability of a substance to become a vapor is increased. Recall that vapor is created when enough heat is applied to the liquid. The temperature at which the liquid becomes vapor is called the boiling point. A liquid turns into a vapor when the vapor pressure (pressure applied by the vapor from the liquid) equals the atmospheric pressure. Decreasing the atmospheric pressure will make it easier for the liquid to turn into a vapor; therefore, this will increase the rate of distillation.
Decreasing the vapor pressure will remove vapor from system. This will make it harder to distill substances. Decreasing temperature will move the system away from the boiling point, thereby decreasing the amount of vapor. Decreasing mole fraction of the substance will decrease the surface area of the substance (at the surface of the solution). Liquid molecules need to be present at the surface to escape the solution and become vapor; therefore, decreasing mole fraction will decrease the amount of vapor.
Example Question #11 : Biochemistry, Organic Chemistry, And Other Concepts
A new student is planning to use thin layer chromatography (TLC) for his research project. After setting up the apparatus the student forgets to place a lid on the TLC jar. He obtains poor results after running the TLC experiment. Which of the following can best explain his bad results?
The open system evaporates the solvent in the solution
The open system prevents the evaporation of the solvent in the solution
The open system prevents the evaporation of the solvent on the TLC plate
The open system evaporates the solvent on the TLC plate
The open system evaporates the solvent on the TLC plate
TLC is a laboratory technique commonly used to separate components of a mixture. Mixtures are placed on the TLC plate (stationary phase), which is then transferred to a jar containing the solvent. The solvent travels through the plate and carries components of the mixture along with it. Based on its properties, each component is dragged to different distances on the plate. The relative distances travelled by each component can be used for separation and identification.
It is important to place a lid on the jar because the solvent will be a volatile substance. An open system will allow for the solvent to evaporate from the TLC plate and reduce the amount of solvent travelling through the plate. The solvent in the solution will evaporate, but it is negligible and inconsequential to the data collected on the TLC plate.
Example Question #3 : Purification Techniques
Which of the following can be determined from thin layer chromatography results?
I. Number of components in the mixture
II. The identity of the components
III. Polarity of components
II only
II and III
I and III
I, II, and III
I and III
Thin layer chromatography (TLC) is used to separate components in a mixture. Components are separated on a TLC plate because each component travels a different distance. The distance travelled depends on several factors. One of those factors is polarity; therefore, TLC can used to determine polarity of substances.
TLC is not useful for identifying substances. Other techniques such as NMR and IR spectroscopy are useful for identifying individual components. The Rf value of a compound can help identify it in a broad sense, but this technique provides only very rudimentary identification capabilities.
Example Question #1 : Thin Layer Chromatography
After performing a TLC experiment, a researcher determines the Rf value of a component to be 2. He also notes that the solvent travelled a distance of 4cm on the plate. What can you conclude about this experiment?
The solvent is not volatile
The reported data does not seem valid
There are multiple components in the mixture
The component travelled a distance of 8cm
The reported data does not seem valid
To solve this problem we need to recall the definition of Rf.
The solvent is typically the most mobile substance on a TLC plate; therefore, it travels the farthest distance. This means that Rf is always less than one because the numerator of Rf is always smaller than the denominator. The question states that the Rf value is 2. Since this is greater than 1, the results don’t seem valid. This value indicates that the solute compounds were able to teravel farther on the TLC plate than the solvent (mobile phase).
Certified Tutor
All MCAT Physical Resources














