All MCAT Physical Resources
Example Questions
Example Question #2 : Vsepr Geometry
Which of the following compounds has a molecular tetrahedral geometry?
Of the available answer choices, only 



Example Question #2 : Vsepr Geometry
What is the molecular geometry of sulfur hexafluoride?
Tetrahedral
Square planar
Octahedral
Trigonal bipyramidal
Octahedral
Sulfur hexaflouride (SF6) is an example of octahedral geometry, as it follows the skeleton of AX6E0 format. A refers to sulfur, X to fluorine, and E to the lone pair electrons.
Square planar has an AX4E2 format, while tetrahedral and trigonal bipyramidal follow AX4 and AX5 formats, respectively.
Example Question #2 : Vsepr Geometry
Which of the following is not the correct geometric configuration for the given molecule?
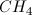





Recall the following relationships between geometry and number of pairs of electrons on the central atom.
2: linear
3: trigonal planar
4: tetrahedral
5: trigonal bipyriamidal
6: octahedral
To visualize the geometry, we need to think of how many electron pairs are on the central atom. Drawing Lewis dot diagrams may be helpful here. None of the answer choices has lone central electron pairs, with the exception of water, so the number of atoms bound to the central atom is the same as the number of central electron pairs.
The only one that does not match up with the correct geometry is SF6, which is actually octahedral since it has six central electron pairs. In a water molecule, the central oxygen has six valence electrons, plus one from each bond with hydrogen, for a total of eight central electrons and four central electron pairs. So, this geometry is a variation on the tetrahedral form (bent), in which two central electron pairs are not bound.
Example Question #3 : Vsepr Geometry
Which of the following molecules will have the smallest bond angles?
In order to determine which molecule will have the smallest bond angle(s), make sure to factor in both the number of atoms around the central atom as well any lone pairs on the central atom. 


Example Question #4 : Vsepr Geometry
The geometry of a certain molecule with the general formula 
Octahedral geometry always corresponds to the 








Example Question #31 : Compounds, Molecules, And Bonds
What is the geometry of 
Trigonal pyramidal
Seesaw
Square planar
Tetrahedral
Square pyramid
Tetrahedral
The central atom, sulfur, is surrounded by four electron groups (oxygen atoms), two of which are double bonded. Also note that the lone pairs are on the oxygen atoms, not the central atom. Thus the molecular geometry is tetrahedral.
Example Question #3 : Vsepr Geometry
According to the VSEPR theory, what is the angle between the two lone pairs in 
According to VSEPR theory, the electron pairs will repel each other as much as possible. Therefore, in the octahedral shape, the lone pairs will be on opposite ends of the molecule, or 
Example Question #1 : Intermolecular Forces
Electronegativity is an important concept in physical chemistry, and often used to help quantify the dipole moment of polar compounds. Polar compounds are different from those compounds that are purely nonpolar or purely ionic. An example can be seen by contrasting sodium chloride, NaCl, with an organic molecule, R-C-OH. The former is purely ionic, and the latter is polar covalent.
When comparing more than one polar covalent molecule, we use the dipole moment value to help us determine relative strength of polarity. Dipole moment, however, is dependent on the electronegativity of the atoms making up the bond. Electronegativity is a property inherent to the atom in question, whereas dipole moment is a property of the bond between them.
For example, oxygen has an electronegativity of 3.44, and hydrogen of 2.20. In other words, oxygen more strongly attracts electrons when in a bond with hydrogen. This leads to the O-H bond having a dipole moment.
When all the dipole moments of polar bonds in a molecule are summed, the molecular dipole moment results, as per the following equation.
Dipole moment = charge * separation distance
A scientist is investigating the polar nature of several compounds. He compares the vapor pressure of water to the vapor pressure of an assortment of low molecular weight hydrocarbons. What is he most likely to find?
The water molecule will likely have lower vapor pressure, owing to stronger intermolecular bonds
The water will likely have higher vapor pressure, owing to its weaker intermolecular bonds
The vapor pressure will vary unpredictably between water and each low molecular weight hydrocarbon
The water will likely have lower vapor pressure, owing to its weaker intermolecular bonds
The water will likely have higher vapor pressure, owing to its stronger intermolecular bonds
The water molecule will likely have lower vapor pressure, owing to stronger intermolecular bonds
The strong polarity of water relative to hydrocarbons means that water will have a more difficult time breaking out of its liquid phase, and into its gas phase to generate a vapor pressure. Substances with a high vapor pressure generally have weaker intermolecular bonds and a lower boiling point.
Example Question #2 : Intermolecular Forces
Boiling point is the temperature a liquid needs to achieve in order to begin its transformation into a gaseous state. Campers and hikers who prepare food during their trips have to account for differences in atmospheric pressure as they ascend in elevation. During the ascent, the decrease in atmospheric pressure changes the temperature at which water boils.
Further complicating the matter is the observation that addition of a solute to a pure liquid also changes the boiling point. Raoult’s Law can be used to understand the changes in boiling point if a non-volatile solute is present, as expressed here.
In this law, 


A scientist is studying a series of compounds at standard conditions. Of the compounds listed below, which is likely to have the highest vapor pressure?
In this example, methane, 
Sodium chloride is a solid salt. Solids do in fact have vapor pressures, but the ionic structure of this salt makes it very low.
Example Question #32 : Compounds, Molecules, And Bonds
Which hydrocarbon has the highest melting point?
Melting points of hydrocarbons are determined by two main factors: length of the carbon chain and degree of saturation. Longer carbon chains will have higher melting points, and chains with more saturated bonds have higher melting points.
Of the given answers, 
All MCAT Physical Resources































