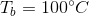All Physical Chemistry Resources
Example Questions
Example Question #1 : Colligative Properties
What is the boiling point of a 100mL solution of water after the addition of 29g of sodium chloride?
Possible Answers:
Correct answer:
Explanation:
We can use the boiling point elevation equation in order to find the new boiling point for the solution.
The change in temperature will be equal to the boiling point elevation constant multiplied by the molality of the solution multiplied by the van't Hoff factor. The van't Hoff factor is a variable that incorporates how many ions the added solute will dissolve into when in solution. Sodium chloride will dissolve into two ions, so the van't Hoff factor is 2.
This means that the boiling point of the water has been elevated by 5.2 degrees, meaning that the boiling point of the solution is 105.2 degrees celsius.
Dimitrios
Certified Tutor
Certified Tutor
Pennsylvania State University-Main Campus, Bachelor of Science, Biochemistry and Molecular Biology. University of California-...
Natalia
Certified Tutor
Certified Tutor
Lomonosov Moscow state university, Master's/Graduate, Biochemistry. Shemyakin-Ovchinnikov Institute of bioorganic chemistry,...
All Physical Chemistry Resources
Physical Chemistry Tutors in Top Cities:
Atlanta Physical Chemistry Tutors, Austin Physical Chemistry Tutors, Boston Physical Chemistry Tutors, Chicago Physical Chemistry Tutors, Dallas Fort Worth Physical Chemistry Tutors, Denver Physical Chemistry Tutors, Houston Physical Chemistry Tutors, Kansas City Physical Chemistry Tutors, Los Angeles Physical Chemistry Tutors, Miami Physical Chemistry Tutors, New York City Physical Chemistry Tutors, Philadelphia Physical Chemistry Tutors, Phoenix Physical Chemistry Tutors, San Diego Physical Chemistry Tutors, San Francisco-Bay Area Physical Chemistry Tutors, Seattle Physical Chemistry Tutors, St. Louis Physical Chemistry Tutors, Tucson Physical Chemistry Tutors, Washington DC Physical Chemistry Tutors
Popular Courses & Classes
Spanish Courses & Classes in New York City, GRE Courses & Classes in Philadelphia, LSAT Courses & Classes in Denver, GRE Courses & Classes in Houston, Spanish Courses & Classes in Houston, GRE Courses & Classes in Atlanta, SSAT Courses & Classes in Boston, MCAT Courses & Classes in San Diego, LSAT Courses & Classes in New York City, SSAT Courses & Classes in Seattle