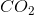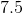All SAT II Chemistry Resources
Example Questions
Example Question #3 : Stoichiometry
When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
___ 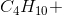



Possible Answers:
Correct answer:
Explanation:
The coefficients of the equation, when balanced, should be 2, 13, 10, and 8. This problem wants to know the sum of the reactants, so only the coefficients on the left side of the reactants should be added. The sum of 2 and 13 is 15, so 15 is the correct answer.
All SAT II Chemistry Resources
Popular Subjects
Math Tutors in Los Angeles, Chemistry Tutors in Los Angeles, French Tutors in Boston, Spanish Tutors in Boston, French Tutors in Atlanta, Calculus Tutors in Boston, SSAT Tutors in Washington DC, Physics Tutors in New York City, Spanish Tutors in Dallas Fort Worth, MCAT Tutors in Houston
Popular Courses & Classes
SAT Courses & Classes in New York City, SAT Courses & Classes in Dallas Fort Worth, GRE Courses & Classes in San Diego, Spanish Courses & Classes in Denver, GRE Courses & Classes in Washington DC, GRE Courses & Classes in Miami, LSAT Courses & Classes in Denver, SAT Courses & Classes in Phoenix, MCAT Courses & Classes in Chicago, SSAT Courses & Classes in Los Angeles
Popular Test Prep
GRE Test Prep in San Diego, MCAT Test Prep in Atlanta, MCAT Test Prep in Philadelphia, GRE Test Prep in Boston, SSAT Test Prep in Boston, GRE Test Prep in Atlanta, GRE Test Prep in Washington DC, ISEE Test Prep in San Francisco-Bay Area, LSAT Test Prep in Boston, SSAT Test Prep in San Francisco-Bay Area