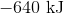All SAT II Chemistry Resources
Example Questions
Example Question #1 : Thermodynamics
Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
Possible Answers:
Correct answer:
Explanation:
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce

Hence, the enthalpy change is 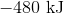
All SAT II Chemistry Resources
Popular Subjects
Chemistry Tutors in Chicago, Statistics Tutors in San Francisco-Bay Area, ISEE Tutors in Seattle, SSAT Tutors in Phoenix, ISEE Tutors in Philadelphia, Reading Tutors in Seattle, Biology Tutors in Seattle, GMAT Tutors in Miami, MCAT Tutors in Los Angeles, GRE Tutors in New York City
Popular Courses & Classes
SSAT Courses & Classes in Seattle, ISEE Courses & Classes in Miami, GRE Courses & Classes in Boston, SAT Courses & Classes in Seattle, SSAT Courses & Classes in San Francisco-Bay Area, GMAT Courses & Classes in Philadelphia, ISEE Courses & Classes in Washington DC, SAT Courses & Classes in Houston, LSAT Courses & Classes in Atlanta, GMAT Courses & Classes in Seattle
Popular Test Prep
SSAT Test Prep in Philadelphia, GMAT Test Prep in Atlanta, SSAT Test Prep in Atlanta, GMAT Test Prep in Seattle, GRE Test Prep in Boston, ISEE Test Prep in Boston, GMAT Test Prep in Washington DC, GRE Test Prep in San Francisco-Bay Area, ISEE Test Prep in Miami, GMAT Test Prep in Miami