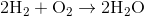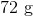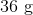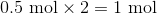All SAT II Chemistry Resources
Example Questions
Example Question #1 : Limiting Reagent
Suppose that 8 grams of of hydrogen gas and 16 grams of oxygen gas are ignited. What is the theoretical yield, in grams, of water? Assume hydrogen has an atomic mass of 1 and oxygen has an atomic mass of 16.
Possible Answers:
Correct answer:
Explanation:
First, convert from grams to moles using the molar masses of the compounds. There are 4 mol of hydrogen gas and 0.5 mol of oxygen gas. Oxygen gas is clearly the limiting reagent (since there is more than twice as much hydrogen gas as oxygen gas), which means that the theoretical yield of water is
Water contains two hydrogen atoms and an oxygen atom, so it has a molar mass of
Hence, 1 mol of water would have a mass of 18 g.
All SAT II Chemistry Resources
Popular Subjects
GMAT Tutors in Seattle, English Tutors in Philadelphia, MCAT Tutors in Seattle, Reading Tutors in Seattle, Computer Science Tutors in Los Angeles, MCAT Tutors in Philadelphia, Computer Science Tutors in Seattle, ACT Tutors in San Diego, Algebra Tutors in Boston, Chemistry Tutors in Chicago
Popular Courses & Classes
GMAT Courses & Classes in Washington DC, SSAT Courses & Classes in Miami, SAT Courses & Classes in Seattle, ACT Courses & Classes in Washington DC, GRE Courses & Classes in Boston, GMAT Courses & Classes in Philadelphia, LSAT Courses & Classes in Dallas Fort Worth, MCAT Courses & Classes in Chicago, ISEE Courses & Classes in Los Angeles, Spanish Courses & Classes in Los Angeles