All SAT II Chemistry Resources
Example Questions
Example Question #1 : Vsepr Theory
Based on VSEPR theory and electron configurations, which of the following molecules is not matched with its correct geometry?
Possible Answers:

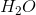



Correct answer:

Explanation:

All SAT II Chemistry Resources
Popular Subjects
Algebra Tutors in Dallas Fort Worth, Physics Tutors in Miami, ISEE Tutors in Chicago, Physics Tutors in Washington DC, Physics Tutors in Seattle, Chemistry Tutors in Houston, GMAT Tutors in Boston, Math Tutors in Boston, SSAT Tutors in San Diego, Reading Tutors in San Diego
Popular Courses & Classes
GRE Courses & Classes in Washington DC, MCAT Courses & Classes in Chicago, Spanish Courses & Classes in Philadelphia, ISEE Courses & Classes in Atlanta, ACT Courses & Classes in Atlanta, Spanish Courses & Classes in Atlanta, SSAT Courses & Classes in Los Angeles, LSAT Courses & Classes in Seattle, SSAT Courses & Classes in Atlanta, SAT Courses & Classes in Los Angeles




