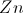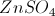All AP Chemistry Resources
Example Questions
Example Question #81 : Reaction Types
In this reaction, electrons are transferred from ___________ to ___________.
hydrogen . . . chlorine
calcium . . . chlorine
chlorine . . . hydrogen
calcium . . . hydrogen
hydrogen . . . calcium
calcium . . . hydrogen
A transfer of electrons indicates an oxidation-reduction reaction. The element losing electrons is oxidized, while the element gaining electrons is reduced.
Calcium is initially a neutral solid, but as a product has a charge of 
Hydrogen initially has a charge of 
We can thus conclude that calcium was oxidized and hydrogen was reduced, indicating that electrons were transferred from calcium to hydrogen.
The charge on chlorine remains 
Example Question #1 : Principles Of Oxidation Reduction Reactions
In the following reaction, which compound is being oxidized?
Hydrogen
No oxidation takes place
Nitrate
Sulfur
Copper
Sulfur
Hydrogen doesn't change. Cu2+ doesn't change (partnered with S2- then with SO42-). Sulfur goes from S2- and S6+(paired with 6 O2- with a 2– charge), showing an oxidation. Nitrogen goes from N5+ to N2+ meaning it was reduced.
Example Question #2 : Principles Of Oxidation Reduction Reactions
Consider the following reaction:
What is the oxidizing agent, and what is the reducing agent?
No redox chemistry occurs
Silver is the oxidizing agent and copper is the reducing agent
Silver is the oxidizing agent and there is no reducing agent
Silver is the reducing agent and copper is the oxidizing agent
Silver is the reducing agent and there is no oxidizing agent
Silver is the oxidizing agent and copper is the reducing agent
Let's break down the reaction into two separate reactions:

We can see that copper loses electrons, while silver gains electrons. Recall that oxidation is loss and reduction is gain, with regard to electrons. Copper is oxidized and silver is reduced.
However, this question asks for the oxidizing agent and reducing agent. Recall that the oxidizing agent is reduced, while the reducing agent is oxidized. Since copper is oxidized, it is the reducing agent. Similarly, since silver is reduced, it is the oxidizing agent.
Example Question #4 : Principles Of Oxidation Reduction Reactions
What does oxidation mean?
Loss of electrons
Gain of electrons
Gain of protons
Loss of protons
Loss of neutrons
Loss of electrons
Think "OIL RIG" Oxidation Is Loss of electrons, Reduction Is Gain of electrons. The loss and gain of electrons is what changes the charge of the atom or molecule. In redox reactions, electrons are transferred. Changing the number of protons changes the identity of the element ,which is not the case. Since chemical reactions involve valence electrons, the nucleus is unchanged.
Example Question #7 : Principles Of Oxidation Reduction Reactions
Which statement about the reaction equation is correct?

This is a hydrolysis reaction

This is a combustion reaction






Example Question #8 : Principles Of Oxidation Reduction Reactions
Oomycetes of the genus Phytophthora are capable of causing economic damage by destroying crops. The name Phytopthora from the Greek phyton = plant, and phthora = destruction literally means plant-destroyer. However, chemists may battle their destructive effects.
The compound named copper(II) carbonate can be used to destroy the plant-destroyer, and can be produced as shown in the following equation:
What is the net ionic equation for the given reaction that produces copper(II) carbonate?
An ionic equation is a chemical equation in which ionic compounds in an aqueous solution are written as dissociated ions.
A complete ionic equation shows all the ions present in the solution (which includes both the reactive and spectator ions), as the dissociated ions. The complete ionic equation for this problem's reaction is:
It is worth noting that in a complete ionic equation, it becomes readily apparent which ions are involved in the oxidation-reduction reaction, and which ions are the spectator ions (those that do not change during the reaction). For example since 




The net ionic equation shows only the reactive ions in solution and the product(s) formed by them. Therefore a net ionic equation can most simply be made by looking at the complete ionic equation and omitting the spectator ions as shown below:
Net ionic equation:
Example Question #81 : Reaction Types
Consider the following reaction:
Which of the following substances is the oxidizing agent?
In an oxidation-reduction reaction, the oxidizing agent is the reactant that accepts electrons, becoming reduced. The oxidizing or reducing agent is always the whole reagent, and is never just the atom in the reagent that is being oxidized or reduced.




Example Question #149 : Reactions And Equilibrium
Put the following acids in order of their INCREASING acid strength: HI, HCl, HBr, HF.
HF, HBR, HI, HCl
HF, HCl, HBr, HI
HI, HBr, HCl, HF
HCl, HBr, HI, HF
HI, HCl, HBr, HF
HF, HCl, HBr, HI
Larger halogen size leads to greater acidity because of weaker H-X interactions.
Example Question #81 : Reaction Types
Put the following acids in order of their INCREASING acid strength: HCl, HS, HBr, H2Se.
H2S, H2Se, HCl, HBr
HBr, H2Se, HCl, H2S
H2Se, HBr, HS, HCl
H2S, HCl, HBr, H2Se
HCl, HS, HBr, H2Se
H2S, H2Se, HCl, HBr
Acid strength increases from left to right across a period and increases going down a group.
Example Question #82 : Reaction Types
Put the following acids in order of their DECREASING acid strength: HOCl, HOBr, HOI, H2O.
HOCl, HOBr, HOI, H2O
H2O, HOI, HOBr, HOCl
HOCl, H2O, HOBr, HOI
H2O, HOCl, HOBr, HOI
HOI, HOBr, HOCl, H2O
HOCl, HOBr, HOI, H2O
Acid strength of an oxy acid increases with increasing electronegativity on the halogen.
Certified Tutor
All AP Chemistry Resources