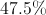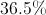All MCAT Physical Resources
Example Questions
Example Question #11 : Stoichiometry And Analytical Chemistry
Which of the following is a molecular formula but not an empirical formula?
An empirical formula is the simplest form of a molecular formula that still retains the ratio of the elements. If a formula can be divided by a whole number, it is a molecular formula and not an empirical formula. A molecular formula is the exact identity of a compound, showing the total number of atoms used to create the compound.
The only given answer that is fully divisible by a whole number is 


Example Question #11 : Stoichiometry And Analytical Chemistry
A given compound is composed of 


To find the empirical formula, use the mass percentage of each element to find mole ratios based on a hypothetical sample of 
We see that there is a 1:1 mole ratio for carbon to hydrogen, making the empirical formula 
The next step will be to find molecular formula by dividing the molar mass of the compound by the molar mass of the empirical formula.
Multiply the subscripts of the empirical formula for each element by six to get the molecular formula: 
Example Question #12 : Stoichiometry And Analytical Chemistry
A compound is found to have a molar mass of 



To find the empirical formula, we must first find the mole ratios by using molar masses for each element:
Rounding these numbers, we get five moles of carbon, ten moles of hydrogen, and two moles of oxygen, making the formula 
We know the molecular mass, given in the question. To find the molecular formula, we need to find the ratio of the mass of the empirical formula to the molecular mass.
The mass of the empirical formula is equal to the molecular mass, meaning that the empirical and molecular formulas are the same.
Example Question #13 : Molecular Weight, Molecular Formula, And Moles
Consider the following molecular formulas:
*The IUPAC name for DEET is N,N-diethyl-meta-toluamide
Which of the following is NOT true?
The combined mass percentage of hydrogen and carbon is ribose is greater than the mass percentage of oxygen
The molecular formula for DEET is the same as its empirical formula
The empirical formula for ribose is
Oxygen represents a larger percentage by mass in ethyl butyrate than in chlorophyll
The combined mass percentage of hydrogen and carbon is ribose is greater than the mass percentage of oxygen
The molecular formula is the same as the empirical formula if it cannot be reduced by any whole number. Any formula containing a single atom of any given element must be an empirical formula as well. The formula for DEET is 
The molecular formula for ribose is 

The other two options require us to calculate pass percentages based on the given molecular formulas.
Although we can look at the formulas for chlorophyll and ethyl butyrate to deduce that oxygen makes up a larger percentage of the latter, we can double check mathematically. In order to find which compound contains oxygen in a larger percentage, divide the molar mass of the oxygen in the compound by the molar mass of the entire compound.
For ethyl butyrate:
For chlorophyll:
We see that it is in fact true that there is a larger percentage of oxygen in ethyl buyrate.
Next, find out if hydrogen and carbon make up the largest mass percentage of ribose by using the same method:
This amounts to 46.7% of the molecular mass, meaning that oxygen must account for the remaining 53.3%. Oxygen thus makes up a greater mass percentage of ribose than hydrogen and carbon combined.
Example Question #19 : Stoichiometry And Analytical Chemistry
What is the empirical formula for a compound that is composed of 53.3% oxygen, 6.7% hydrogen, and 40% carbon?
Given the percentages by mass of the compound, we can convert the percentages to grams by considering a 100-gram sample of the compound. The next step involves dividing each mass by the element's molar mass.
By dividing each value by the smallest molar quantity, we can determine the ratio of elements in the compound.
As a result, the empirical formula for the compound is 
Example Question #21 : Stoichiometry And Analytical Chemistry
Manganese forms a number of oxides, one of which is composed of 72% manganese by mass. Which of the following is the formula for this oxide?
Given that the molar mass of oxygen is about 16g, and molar mass of manganese is about 55g, 
165g/229g = 0.72
So, the ratio of manganese to oxygen in this compound is 72% manganese by mass.
Example Question #22 : Stoichiometry And Analytical Chemistry
What is the mass percentage of carbon in glucose (C6H12O6)?
25%
It depends on the amount of glucose.
40%
96%
40%
In order to find the mass percentage of an atom in a molecule, start by finding the total mass of one mole of the molecule. Glucose has 180 grams per mol.
Next, we determine the mass of the carbon atoms in one mole of the molecule. One carbon mole has a mass of 12 grams. Multiplied by the six carbon atoms in glucose gives a mass of 72 grams.
Finally, we divide the mass of carbon by the mass of the molecule.
So, 40% of glucose's mass is made up of carbon.
Example Question #23 : Molecular Weight, Molecular Formula, And Moles
What is the mass percentage of aluminum in aluminum (III) oxide?
Aluminum oxide has the formula 
Aluminum has a molecular weight of 

The mass percentage is given by the mass of aluminum divided by the total molecular weight.
Example Question #24 : Molecular Weight, Molecular Formula, And Moles
What is the percentage by weight of sodium in sodium sulfate?
Sodium sulfate is given by the formula:
To find the percentage by weight, we will need to divide the mass of sodium in the molecule by the total molecular mass.
Convert the ratio to a percentage.
Example Question #24 : Molecular Weight, Molecular Formula, And Moles
Which of the following samples contains a larger mass of hydrogen?
The samples contain equal amounts of hydrogen
We must know the density of each compound in order to solve
Start by calculating the percentages of hydrogen in each compound by using molar mass ratios.
Next, take the percentages of the given sample masses to determine the total mass of hydrogen in each.
We see that there is a larger mass of hydrogen in the hydrogen cyanide sample.
Certified Tutor
All MCAT Physical Resources